O Effective Nuclear Charge
Electron Shielding and Effective Nuclear Charge. This chemistry video tutorial explains how to calculate the effective nuclear charge of an electron using the atomic number and the number inner shell electr.

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration
For 2nd and 3rd row d metals spin-orbit coupling is significant.

O effective nuclear charge. How do you calculate effective nuclear charge. The µspin-only formula applies only to 1st row d metal ions. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the.
Core electrons efficiently shield electrons in the outermost principal level from nuclear charge but outermost electrons do not efficiently shield one another from the nuclear charge. ENC effective nuclear charge of protons in nucleus of shielding inner shell electrons. So be able to do this calculation.
The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. Effective nuclear charge increases going left to right across a row of the periodic table. Effective nuclear charge refers to the charge that the outermost valance electron have.
Note the value is a charge and contains no units. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. Screening Percentages Based on Slater Effective Nuclear Charge as a Versatile Tool for Teaching Periodic Trends Journal of Chemical Education volume.
True O False Which of the following statements is false. With atoms the effective nuclear charge refers to the net charge experienced by an atoms outmost electrons. This calculator is based on the Slaters rule of calculating effective nuclear charge.
Core electrons pulled in tightly Valence electrons held less tightly Shielding effect reduces the full nuclear charge of outer electrons. Also the electron or multi-electron takes into account the number of. 72 EFFECTIVE NUCLEAR CHARGE The NET positive charge experienced by an electron.
The effective nuclear charge may be approximated by the equation. Where Z is the atomic number and S is the number of shielding electrons. Z eff Z - S.
Learn about effective nuclear charge and periodic trends. If an electron is far from the nucleus ie if the distance r between the nucleus and the electron is large then at any given moment many of the other electrons will be between that electron and the nucleus Figure PageIndex1. This online chemistry calculator calculates the effective nuclear charge on an electron.
Review atoms the periodic. You can calculate effective nuclear charge if you know the number of inner electrons and the number of protons of an atom both which can be found either from the periodic table or from online resources. Hence the electrons will cancel a portion of the positive charge of the nucleus and thereby decrease.
BASED On The PERIODIC. Electrons in an atom can shield each other from the pull of the nucleus. Hence the effective nuclear charge for oxygen atom is 455.
The equation for calculating nuclear charge is Zeff Z - S where Zeff is the effective nuclear charge Z is the number of protons and S is. The effective nuclear charge of an atom is calculated as the decreases in the expected nuclear charge of valence electronsdue to the screening by other electrons. Answer 1 of 3.
Nuclear charge and effective nuclear charge are two different values that are calculated regarding atoms of chemical elements. For oxygen atom the electronic config is. The stronger pull higher effective nuclear charge experienced by electrons on the right side of the periodic table draws them closer to the nucleus making the atomic radii smaller.
Write down the electronic configuration. Nuclear charge is the total charge of a nucleus. The size of an anion is greater compared to its parent atom because formers effective nuclear charge is lesser than that of latter.
Effective nuclear charge is the net charge that an outermost shell electron experiences. This effect called the shielding effect describes the decrease in the attraction between an electron and the nucleus in any atom with more than one electron shell. The effective nuclear charge of the 3s1 electron in the sodium atom is 22.
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the. The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom.
It wants you to think of the nucleus plus all the non-outer-shell electrons as a. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. 72 Effective Nuclear Charge.
With increasing effective nuclear charge Zeff the orbitals of an atom are increasingly destabilized. Follow the steps below to calculate effective nuclear charge by the Slaters rule. Of protons in the nucleus - No.
The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. The math equation below determines the effective or actual nuclear charge and as mentioned before ENC explains all of the trends in the periodic table. O The effective nuclear charge can be thought of as the true nuclear charge minus a screening constant due to the other electrons in the atom.
Effective nuclear charge is a concept that helps to understand how strongly the outer-shell electrons are held by the atom. I found on wikipedia that the effective nuclear charge can be calculated by the formula.

Effective Nuclear Charge Wikipedia Effective Nuclear Charge Nuclear Chemistry

How Does Atomic Size Effective Nuclear Charge And Hybridization Affect Electronegativity Chemistry In 2021 Effective Nuclear Charge Chemistry Atom
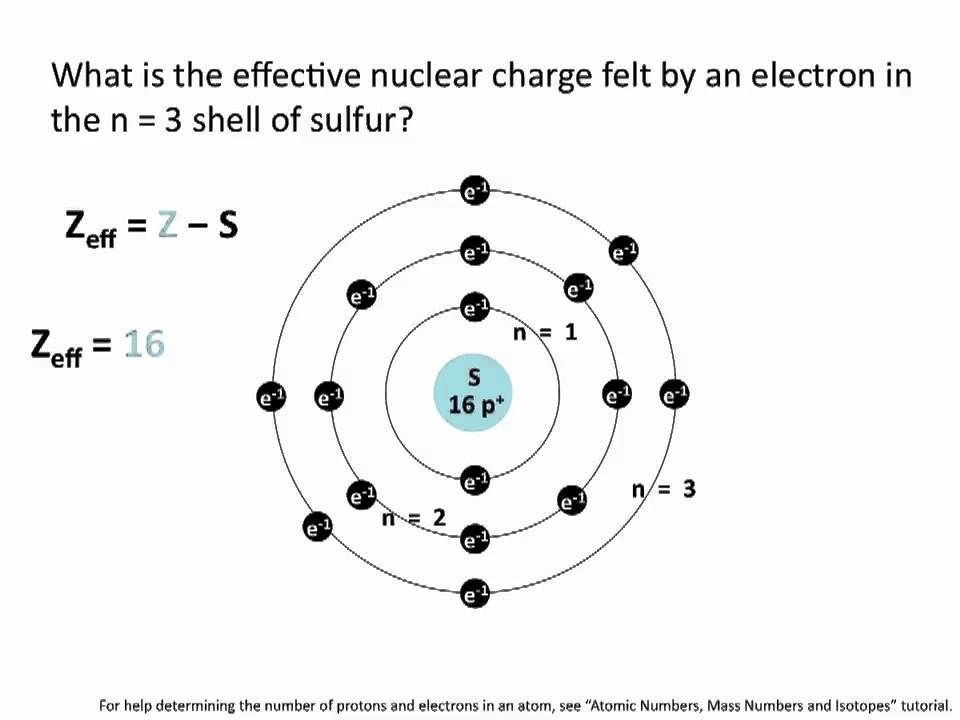
Effective Nuclear Charge Chemistry Tutorial Youtube Effective Nuclear Charge Chemistry Jokes Chemistry

Effective Nuclear Charge Wikipedia Effective Nuclear Charge Nuclear Chemistry

Effective Nuclear Charge And The Shielding Effect Effective Nuclear Charge Ionization Energy Electron Affinity

7 2 Shielding And Effective Nuclear Charge Chemistry Libretexts In 2021 Effective Nuclear Charge Chemistry Neon Atom

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Electron Configuration Neon Atom

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration

Shielding Effect And Effective Nuclear Charge Effective Nuclear Charge Chemistry Activities Nuclear

The Shielding Effect And Effective Nuclear Charge Introduction To Chemistry Electron Configuration Effective Nuclear Charge Chemistry Textbook

Neon S Atomic Radius Is 38 Pm Effective Nuclear Charge Element Chemistry Electron Affinity

Effective Nuclear Charge Happy Students Chemistry

Pin By Faris Barbarossa On الكيمياء Effective Nuclear Charge Chemistry College Chemistry

1 Shielding Effect Effective Nuclear Charge Z Eff Experienced By An Electron Is Less Than T Effective Nuclear Charge Ionization Energy Electron Configuration
Posting Komentar untuk "O Effective Nuclear Charge"