Effective Nuclear Charge Of Br
The chemical symbol for Bromine is Br. See all 8 effective nuclear charges.

Chapter 4 Periodic Trends Of The Elements Ppt Download
Also the electron or multi-electron takes into account the number of shielding electrons that surrounds the nucleus.
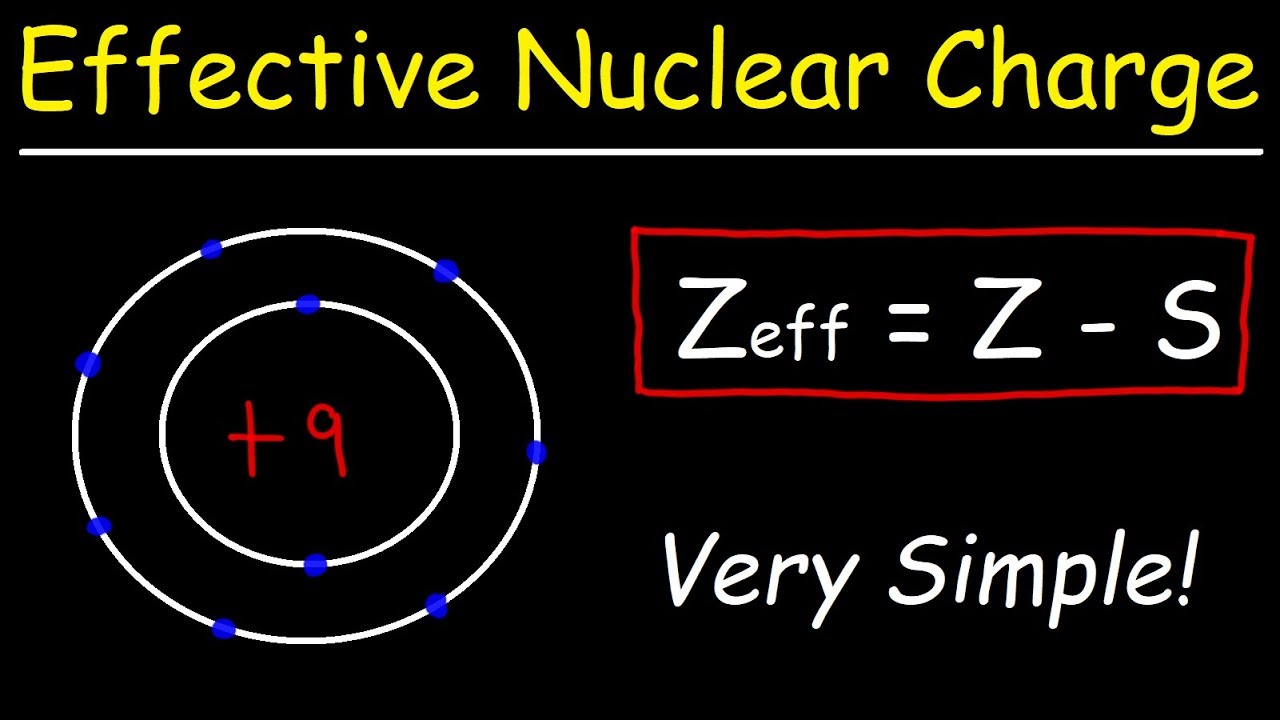
Effective nuclear charge of br. The effective nuclear charge is the actual amount of positive charge experienced by an electron in a polyelectronic atom. Z Z-a is given by number of protons Z number of inner electrons a depends on periodic property is a atomic number is Rafa Muñoa. Where Z is the atomic number and S is the number of shielding electrons.
Write down the electronic configuration. Where μ dipole moment. Effective Nuclear Charge Concept and Applications Rafa Muñoa Lizardi Institutua Zarautz 2.
Updated February 21 2020. Bromine is the third-lightest halogen and is a fuming red-brown liquid at room temperature that evaporates readily to form a similarly coloured gas. For bromine Z eff 35 - 28 7.
The effective nuclear charge is the net positive charge experienced by valence electrons. This online chemistry calculator calculates the effective nuclear charge on an electron. S 35 - 7 28 7.
Calculate the effective nuclear charge at the periphery of nitrogen atom when an extra electron is added in the formation of anion. Electrons that are closer to the nucleus which are referred to as inner or core electrons. Q magnitude of charge.
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the. Z eff ζ n. Notice that Z e f f Z only for hydrogen Figure 72.
The Effective Nuclear Charge fo Br is 7. Also calculate the effective nuclear change of N atom. Conceptual Map effective nuclear charge Z net positive charge outer electrons is the that attracts equation.
84 76 ratings Problem Details. Effective nuclear charge refers to the charge that the outermost valance electron have. In this topic we are going to discuss the effective nuclear charge and how to calculate it.
Zeff Z S where Z is the atomic number and S is the number of shielding electrons. Z eff Z - S. Ffor example the effective nuclear charge on the 2p orbital in sodium would be 7 because the total nuclear charge is 11 but the 4 electrons in the 1s and 2s orbitals screen 4 lead to an.
Calculate the effective charges on the Br atom of the HBr molecule in units of the electronic charge e. The difference between the full nuclear charge Z and the screening effect of the inner two electrons is called the effective nuclear charge or Z eff. For nitrogen Electronic configuration 1 s 2 2 s 2 2 p 3.
The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. μ q r. This chemistry video tutorial explains how to calculate the effective nuclear charge of an electron using the atomic number and the number inner shell electr.
The effective nuclear charge experienced by an electron is also called. Boron The next element after beryllium is boron. The effective nuclear charge may be approximated by the equation.
At r 0 the positive charge experienced by an electron is approximately the full nuclear charge or Z e f f Z. Z eff Z S where Z is the atomic number and S is the number of shielding electrons. Anne Marie Helmenstine PhD.
Introduction to Effective Nuclear charge. The Effective Nuclear Charge of Se is 6. The effective nuclear charge is determined by subtracting from the number of protons in the nucleus Z the number of inner core IC electrons that shield the valence electron from the nucleus.
An analysis of the effective nuclear charge of ions based on linear relationships of the reciprocal of an ionic radius or the square root of ionisation energy and the nuclear charge of the ion has. The two inner electrons in the 1s orbital screen the third electron from the full effect of the nuclear 3 charge. It can be approximated by the equation.
R bond length. Dipole moment μ is defined as the product of charge separation bond length and the magnitude of charges. This means that Pb will have the highest effective nuclear charge atomic number82 followed by Sn atomic number50 Br atomic number35 Ga atomic number31 and lastly Ti atomic number22.
Z eff Z - IC. The valence shell according to the configuration is 4 and valence electrons are 7. The effective nuclear charge for any subshell is the total positive charge of the nucleus minus the total negative charge of the previous subshells.
721 1 Z e f f Z. This calculator is based on the Slaters rule of calculating effective nuclear charge. The effective nuclear charge is the net positive charge experienced by valence electrons.
Follow the steps below to calculate effective nuclear charge by the Slaters rule. In general for any many-electron atom any particular electron will always be screened from the nucleus to some extent by the remaining electrons. The effective nuclear charge is then the net electric field that a particular electron experiences.
The effective nuclear charge on the added electron in F-atom in the formation of negative ion is. Bromine is a chemical element with atomic number 35 which means there are 35 protons and 35 electrons in the atomic structure. It can be approximated by the equation.
The effective nuclear charge holding a 2s electron to the nucleus is thus nearly 2 about twice the value for lithium and the 2s electron clouds are drawn closer to the center of the atom. The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. The term effective is used because the shielding effect of negatively charged electrons prevent higher orbitals from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer.
At intermediate values of r the effective nuclear charge is somewhere between 1 and Z.

How To Calculate Effective Nuclear Charge Slide Share

Calculate The Effective Nuclear Charge Of The Last Electron In An Atom The Electronic Configuration Is 1s 2 2s 2 2p 6 3s 2 3p 5

04 Periodic Trends And Effective Nuclear Charge Supplement
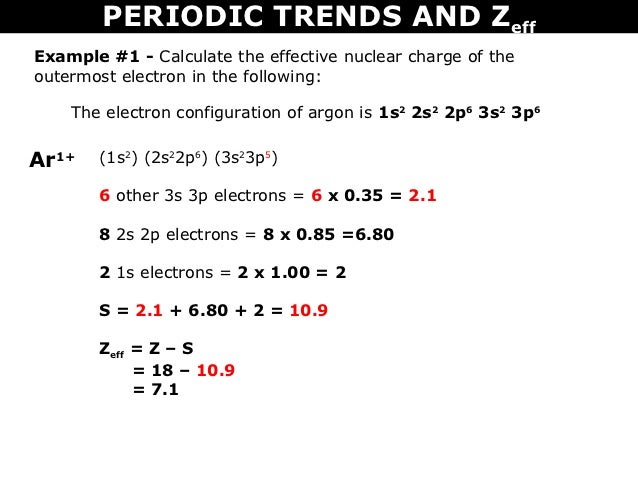
Tang 10 Periodic Trends And Zeff

Trick For Slater S Rule Calculation Of Screening Constant And Effective Nuclear Charge Youtube

1 1 2 Effective Nuclear Charge Chemistry Libretexts
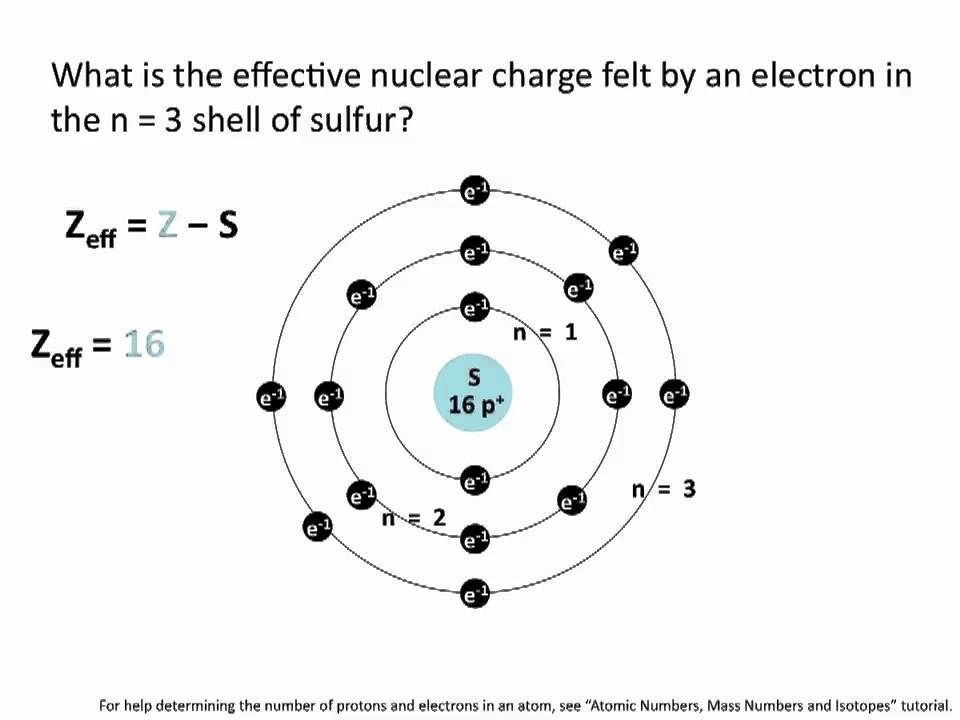
Effective Nuclear Charge Chemistry Tutorial Youtube

How To Calculate The Effective Nuclear Charge Of An Electron Youtube
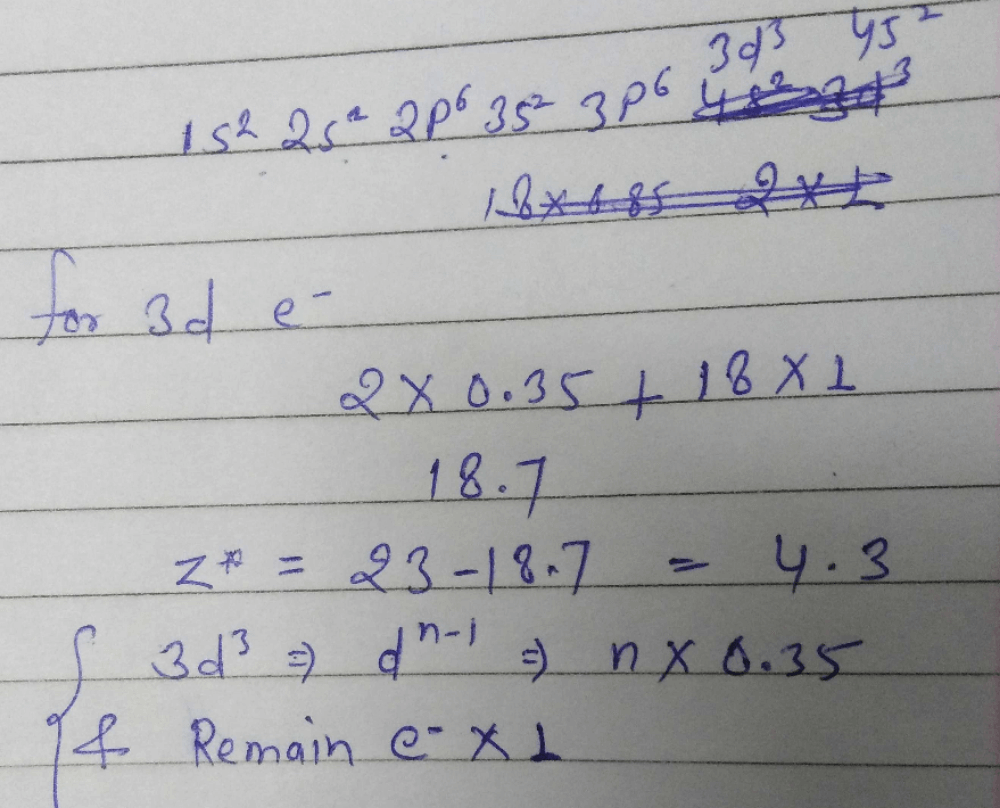
Effective Nuclear Charge For 3d Electron In Vanadium Atomic Number 23 According To Slaters Rule Is Correct Answer Is Between 4 2 4 4 Can You Explain This Answer Edurev Chemistry Question
Shielding Effect N N N Effective Nuclear Charge
Effective Nuclear Charge Periodic Trends

Chapter 4 Periodic Trends Of The Elements Ppt Download

Mcat General Chemistry Ch 2 The Periodic Table Flashcards Quizlet

Posting Komentar untuk "Effective Nuclear Charge Of Br"