Effective Nuclear Charge Of Tin
The effective nuclear charge is the actual amount of positive nuclear charge experienced by an electron in a polyelectronic atom. Follow the steps below to calculate effective nuclear charge by the Slaters rule.
Zeff the effective nuclear charge Z denotes the number of protons existing in the nucleus S average amount of density between the nucleus and the electron.

Effective nuclear charge of tin. What is EFFECTIVE nuclear charge. More generally for carbon valence. Jan 28 2021 0725 PM.
A 1s electron in carbon a 3s electron in silicon a 3s electron in. So its useful to be able to predict effective nuclear charge. The effective nuclear charge may be taken as one.
Predict the possible ions of tin Sn and explain your reasoning using electron configurations. To create the Sn2 ion the electrons of the unfilled 5p shell are. Effective Nuclear Charge of Tin 1 answer below Use Slaters rules to calculate the effective nuclear charge experienced by electrons in the Tin 4d orbital.
The sharge of an element. Also we solve this to find the effective charge of the electron. This calculator is based on the Slaters rule of calculating effective nuclear charge.
This online chemistry calculator calculates the effective nuclear charge on an electron. Screening Percentages Based on Slater Effective Nuclear Charge as a Versatile Tool for Teaching Periodic Trends Journal of Chemical Education volume. Higher energy electrons can have other lower energy electrons between the electron.
Ffor example the effective nuclear charge on the 2p orbital in sodium would be 7 because the total nuclear charge is 11 but the 4 electrons in the 1s and 2s orbitals screen 4 lead to an. A 1s B 3p C 3d D 5s E 5p. How is effective nuclear charge of a valence electron affected - if at all - when a cation is formed.
The most likely ions of tin Sn are Sn2 and Sn4. Effective nuclear charge Clementi - 5s. The effective nuclear charge on an electron is given by Z Z S where Z is the atomic number of the atom and S is the screening constant.
Electron binding energies K tin. The resulting electronegativity of the sp 2 carbon is higher than for the sp 3 carbon. Slaters rules give a simple approximation of effective nuclear charge that works pretty well.
I All electrons in the ns np and nd orbitals n orbital in which the e View the full answer. Moreover you can use this formula to find the effective nuclear charge for an electron in lithium especially the. Answered Sep 11 2016 by Jamie.
The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. Effective nuclear charge Clementi - 5p. The effective nuclear charge for any subshell is the total positive charge of the nucleus minus the total negative charge of the previous subshells.
We have to find in which subshell electrons experience the lowest effective nuclear charge. Atomic weights of the elements 2009 IUPAC Technical Report. The charge felt by the valence electrons.
Calculate the effective nuclear charge for an electron in each of the following orbitals of tellurium Te using Slaters Rules. The charge that actually matters. Were being asked to determine the subshell in the tin atom that experiences the lowest effective nuclear charge.
Effective nuclear charge Clementi - 6p. Write down the electronic configuration. Recall that the effective nuclear charge is the force exerted by the nucleus onto an electron.
The effective nuclear charge may be approximated by the equation. Effective Nuclear Charge of Tin. Outer most sub-shell is 5p.
We know that the electron in outermost shell experience the lowest effective nuclear charge. Electrons that are further away from the nucleus have a lower effective nuclear charge. Arrange these electrons in order of decreasing effective nuclear charge.
The term effective is used because the shielding effect of negatively charged electrons prevent higher orbitals from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer. Z eff Z - S. Use the concepts of effective nuclear charge shielding and value of the valence orbital to explain the trend in.
Based on the last section we can expect that. Wieser Michael E and Tyler B. Effective nuclear charge is really important because it determines the size and energy of orbitals which determine most properties of atoms.
Asked Sep 11 2016 in Chemistry by LuckE1. Screening Percentages Based on Slater Effective Nuclear Charge as a Versatile Tool for Teaching Periodic Trends Journal of Chemical Education volume 78 number 5 2001 pp. A tin atom has 50 electrons.
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. We are given that a tin has 50 electrons. Predict the possible ions of tin Sn and explain your reasoning using electron configurations.
Electrons in the _____ subshell experience the lowest effective nuclear charge. Effective nuclear charge Clementi - 6s. Effective nuclear charge is a very important concept in chemistry and is the basis for the qualitative explanation of many observed chemical and physical properties including several periodic trends.
Electrons in which sublevel of this atom would experience the lowest effective nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. Following the same line of argument the effective nuclear charge for an acetylenic carbon sp is much higher than that of the sp 2 carbon based on two unshielded protons.
Chemistry questions and answers. Hence it seems to me to be of significant importance that these values are accurately determined not only for neutral atoms but for monoatomic ions too. By multiplying the Coloumbs law constant k 90 x 109 N m2 C2 by q1 the effective nuclear charge and q2 the charge of the electron and dividing by the radius of the atom squared we can find F which is the force of attraction between the nucleus and the outer electron.
Use Slaters rules to determine S. A tin atom has 50 electrons. Electronic configuration is given by.
Where Z is the atomic number and S is the number of shielding electrons.
Solved Question 1 The Transition From The N 7 To The N 2 Chegg Com

Solved 1 A Tin Sn Atom Has 50 Electrons Electrons In Chegg Com

How To Use Slater S Rule To Estimate The Effective Nuclear Charge Youtube
Solved 12 A Tin Atom Has 50 Electrons Electrons In The Chegg Com
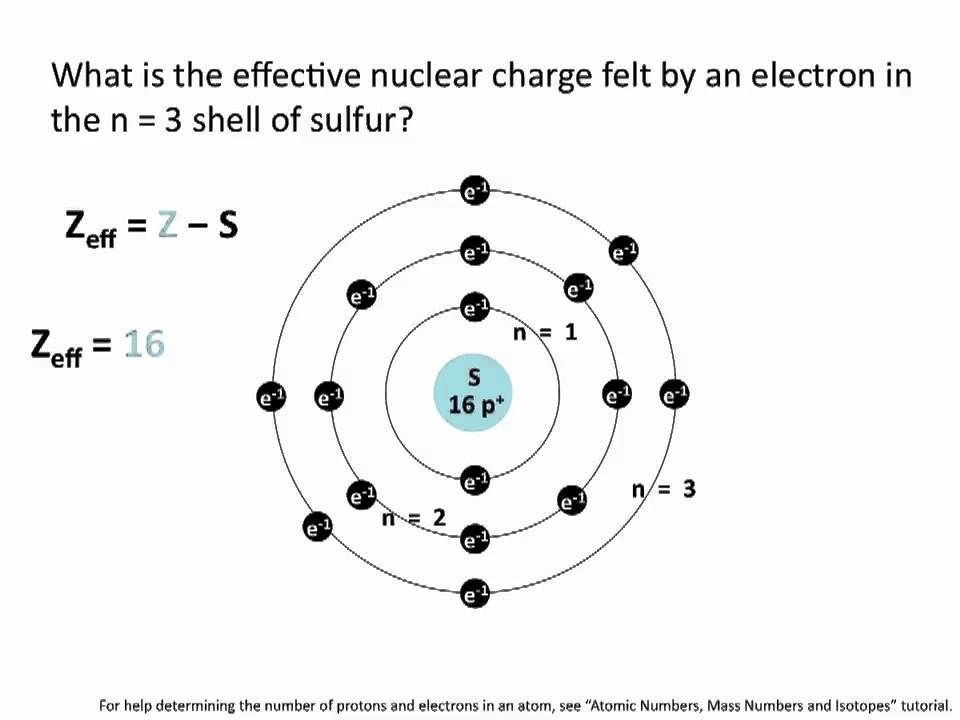
Effective Nuclear Charge Chemistry Tutorial Youtube
Solved Calculate The Effective Nuclear Charge On A 5s 5p Chegg Com
Tin The Periodic Table At Knowledgedoor
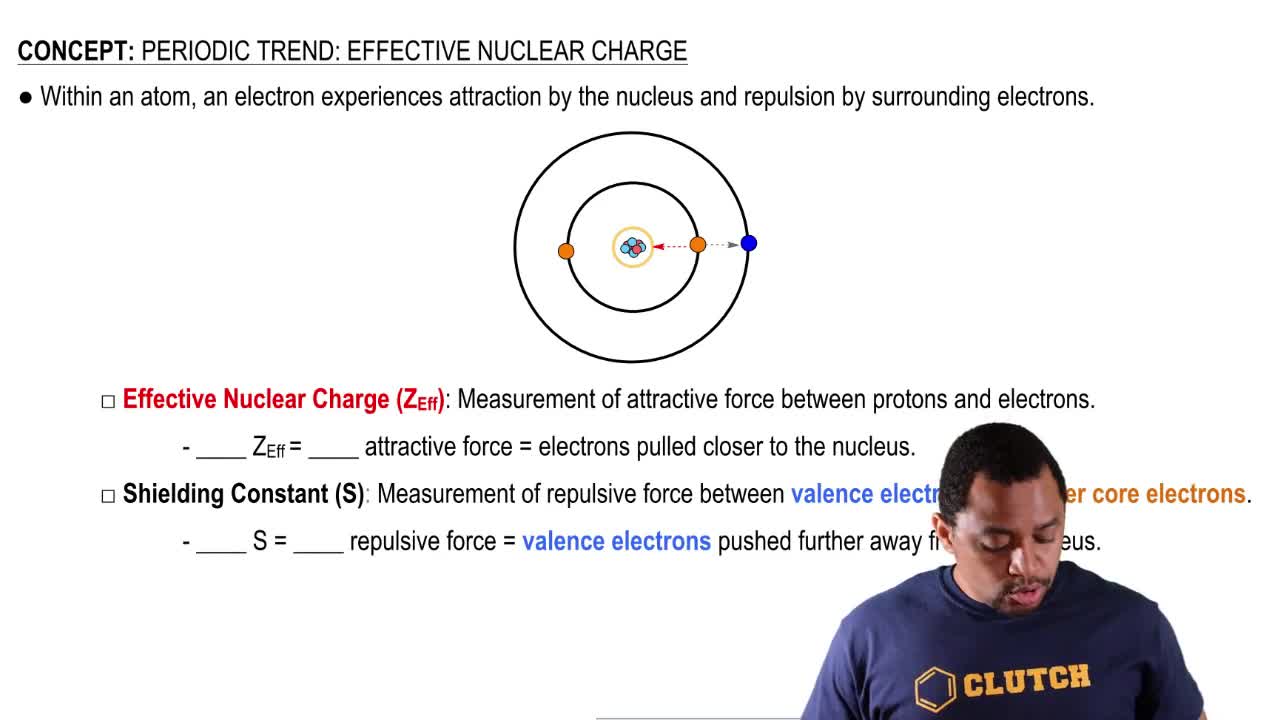
A Tin Atom Has 50 Electrons Electrons In Clutch Prep

Chapter 4 Periodic Trends Of The Elements Ppt Download

1 1 2 Effective Nuclear Charge Chemistry Libretexts

A Tin Atom Has 50 Electrons Electrons In Clutch Prep
Tin The Periodic Table At Knowledgedoor

Webelements Periodic Table Periodicity Effective Nuclear Charge Clementi 6p Periodic Table Gallery



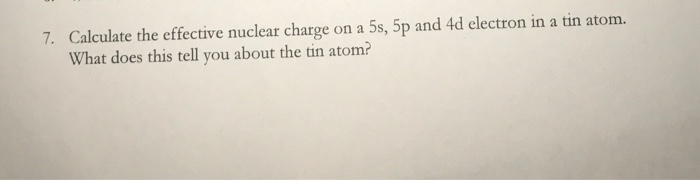
Posting Komentar untuk "Effective Nuclear Charge Of Tin"