Effective Nuclear Charge Of Si
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect of. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons.

The Increasing Order Of Effective Nuclear Charge In Na Ai Mg And Si Atoms Youtube
The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom.

Effective nuclear charge of si. Mg has 2 valence electrons Al has 3 Si has 4 P has 5 S has 6 Cl has 7 while argonAr has eight valence electrons. The effective nuclear charge on an electron is given by the following equation. The effective nuclear charge may be approximated by the equation.
Higher the Effective Nuclear Charge ZEff greater the attractive force which results in electrons being pulled closer to the nucleus. 73 SIZES OF ATOMS. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge.
Effective Nuclear Charge Definition. The nuclear charge of cobalt Co is 27 the same as the atomic number and the number of protons. If you want to know the effective nuclear charge Zeff that requires a more complicated.
Effective Nuclear Charge Trend. Configuration of two elements are. Z is the number of protons in the nucleus.
Si z 14 Ne10 3s2 3p2. Higher the Shielding Constant S greater the repulsive force between valence and inner core electrons which results in valence electrons pushed away from the nucleus. Z eff Z S.
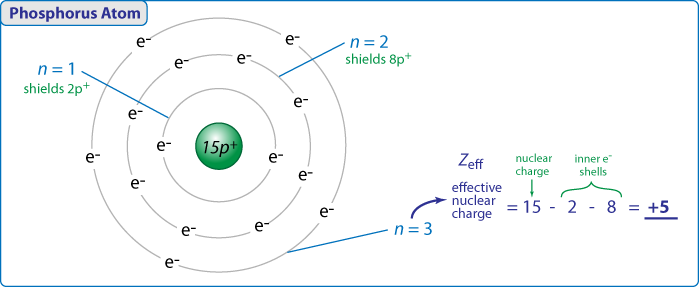
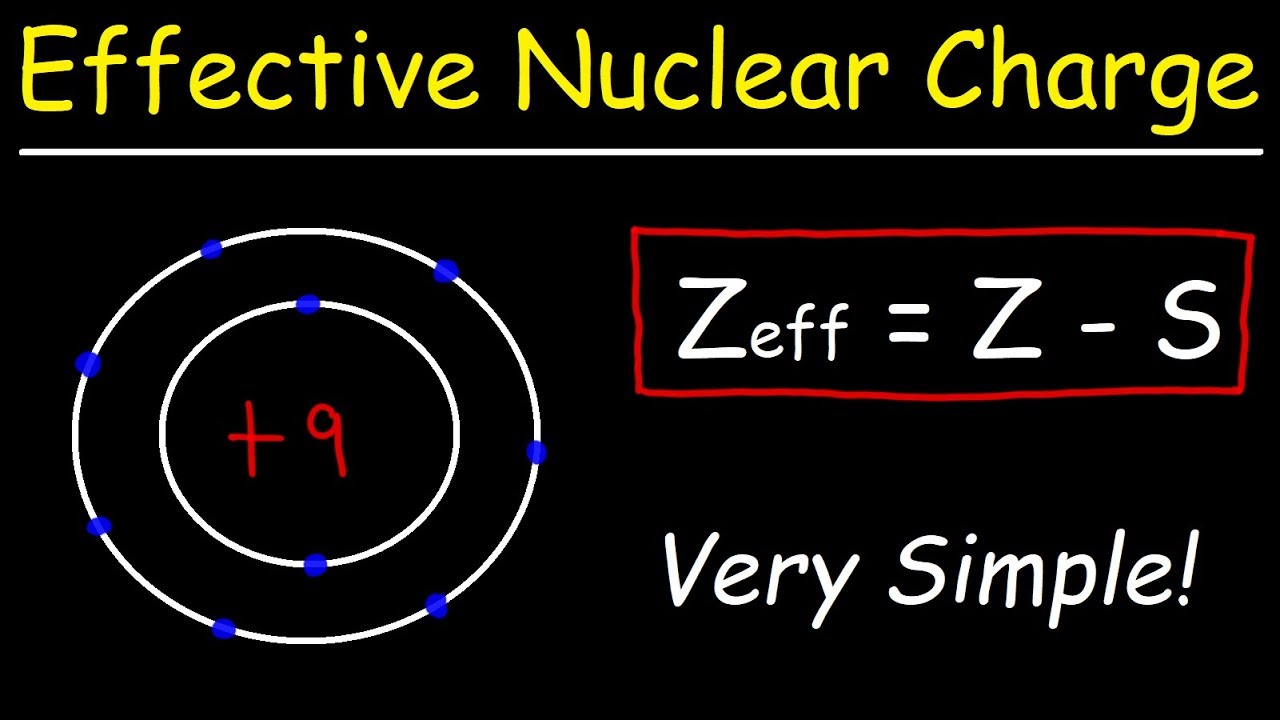
Z eff Z - S. Updated February 21 2020. Where Z eff is the effective nuclear charge.
See all 11 effective nuclear charges. Z eff Z S. The unpaired electrons in silicon Si will experience more effective nuclear charge because the atomic number of the element Si is more than that.
Z eff ζ n. 73 Sizes of atoms As we move down a group the atoms become larger. Where Z is the atomic number and S is the number of shielding electrons.
BASED On The PERIODIC RECURRENCE Of PROPERTIES Periodic Trends In 73 Sizes of atoms and ions. What is the effective nuclear charge felt by a 4p electron of bromine. Follow the steps below to calculate effective nuclear charge by the Slaters rule.
Since Z is dimensionless so is Z e f f. However effective nuclear charge Z e f f e is not and can for instance be expressed in coulombs in the SI system e 1602 176 634 10 19 C. Nuclear charge is defined as the net positive charge experienced by an electron in the orbital of a multi-electron atom.
Answer 1 of 3. Write down the electronic configuration. Hence silicon has a larger nuclear charge of 14 than aluminium which has a nuclear charge of 13.
Note the value is a charge and contains no units. I The electron s present in the 2s orbital will experience greater nuclear charge being closer to the nucleus than the electron. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the.
The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. 1s 2 2s 2p 8 3s 3p 8 3d 10 4s 4p 7 Then write out an equation for the screening constant according to the appropriate Rule - 3 or 4. An effective nuclear charge is defined as the net positive charge of the valence electrons of an atom.
Therefore nuclear charge of period 3 elements also increases. The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. Nuclear Charge Effective Nuclear Charge.
Group the electrons in the following way. 1 the effective nuclear charge Z eff increases as you move to the right making the force holding the electron to the nucleus greater and 2 the distance between the valence electrons and the nucleus decreases as you move up this also makes the force holding the electron to the nucleus greater. Al z 13 Ne10 3s2 3p1.
S is the shielding constant. Thus the electrons in the 3p orbital of silicon will experience a. Z e f f Z σ where σ is the shielding constant of the nucleus empirical dimensionless parameter.
First write out the electronic structure in the format of the first rule. The following formula is used to calculate an effective nuclear charge. The effective nuclear charge of the 3s1 electron in the sodium atom is 22.
This chemistry video tutorial explains how to calculate the effective nuclear charge of an electron using the atomic number and the number inner shell electr. 72 Effective Nuclear Charge. The closer the orbital the greater is the nuclear charge experienced by the electron s in it.
Moving across the period the atomic number increases due to the increase of the number of protons in the nucleus. Effective Nuclear Charge. Due to INCREASING value of n.
An analysis of the effective nuclear charge of ions based on linear relationships of the reciprocal of an ionic radius or the square root of ionisation energy and the nuclear charge of the ion has.
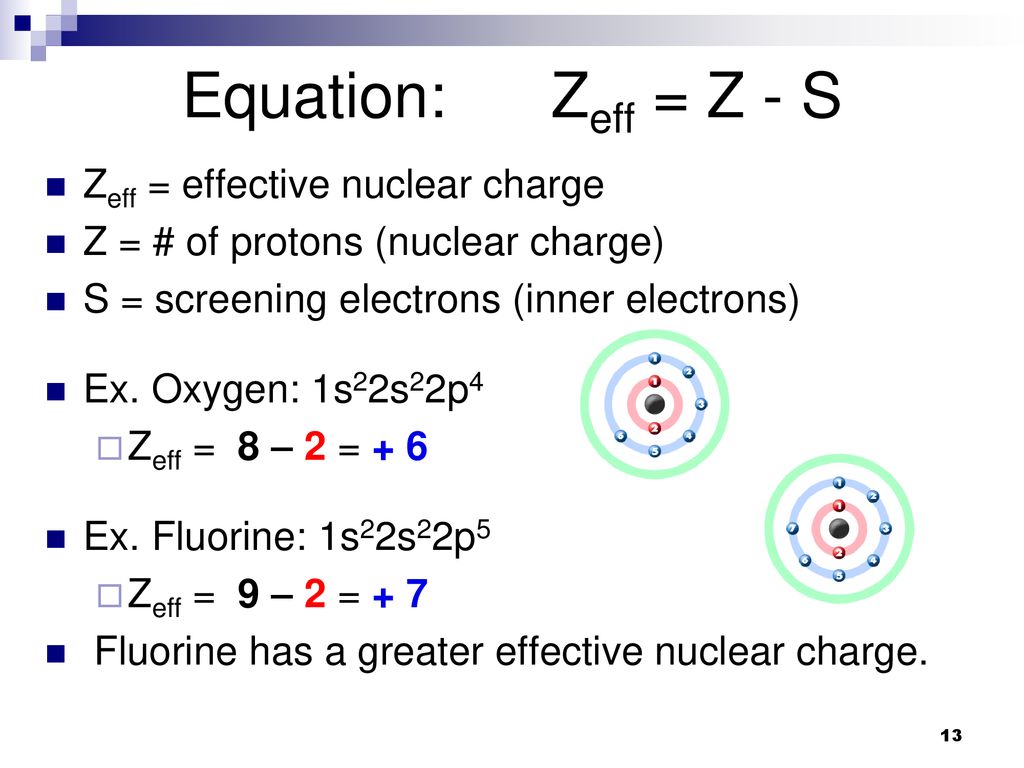
How To Calculate Effective Nuclear Charge Slide Share

What Is Effective Nuclear Charge And How To Calculate It Slater S Rules
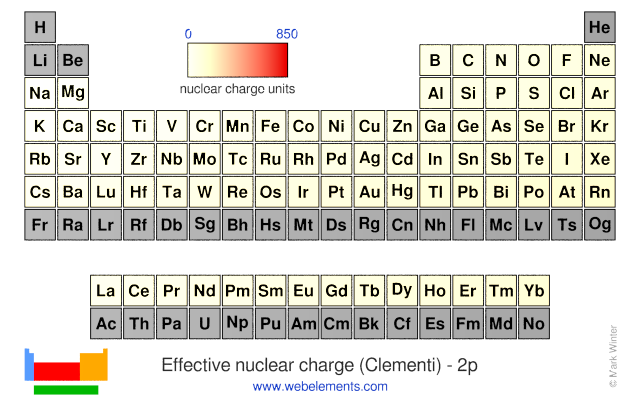
Webelements Periodic Table Periodicity Effective Nuclear Charge Clementi 2p Periodic Table Gallery
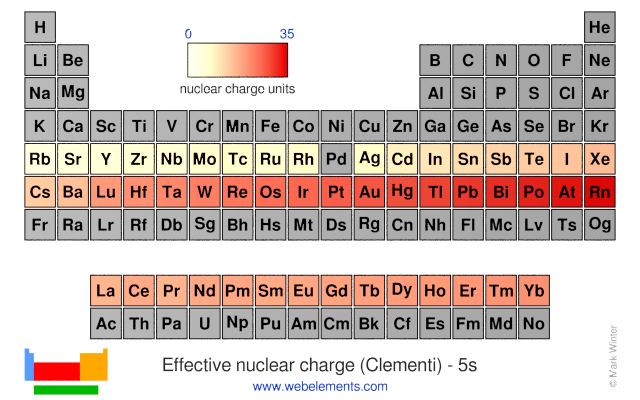
Webelements Periodic Table Periodicity Effective Nuclear Charge Clementi 5s Periodic Table Gallery

Effective Nuclear Charge Periodic Table Slide Share

Effective Nuclear Charge Periodic Table Slide Share

Unit 8 The Periodic Table Trends Ppt Download

Chapter 6 The Periodic Table Jennie L Borders
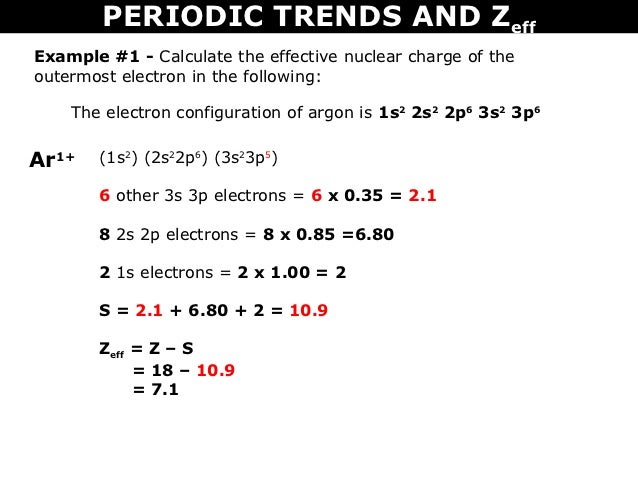
Tang 10 Periodic Trends And Zeff
How To Calculate Effective Nuclear Charge Slide Share
How To Calculate The Effective Nuclear Charge Of An Electron Youtube

Effective Nuclear Charge Chemistry Tutorial Youtube

How To Calculate Effective Nuclear Charge Slide Share

Ppt Effective Nuclear Charge Z Eff Powerpoint Presentation Free Download Id 2630630
Posting Komentar untuk "Effective Nuclear Charge Of Si"