K Effective Nuclear
Where Z is the atomic number and S is the number of shielding electrons. The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom.

Pin By Pieter De Kooker On Chemistry Chemistry Activities Moving
The effective nuclear charge often symbolized as Zeff or Z is the net positive charge experienced by an electron in a multi-electron atom.

K effective nuclear. Decreases fast fission of U238. The effective nuclear charge is the actual amount of positive charge experienced by an electron in a polyelectronic atom. It is the positive charge from a nucleus that an electron feels in an atom with more than one electron present.
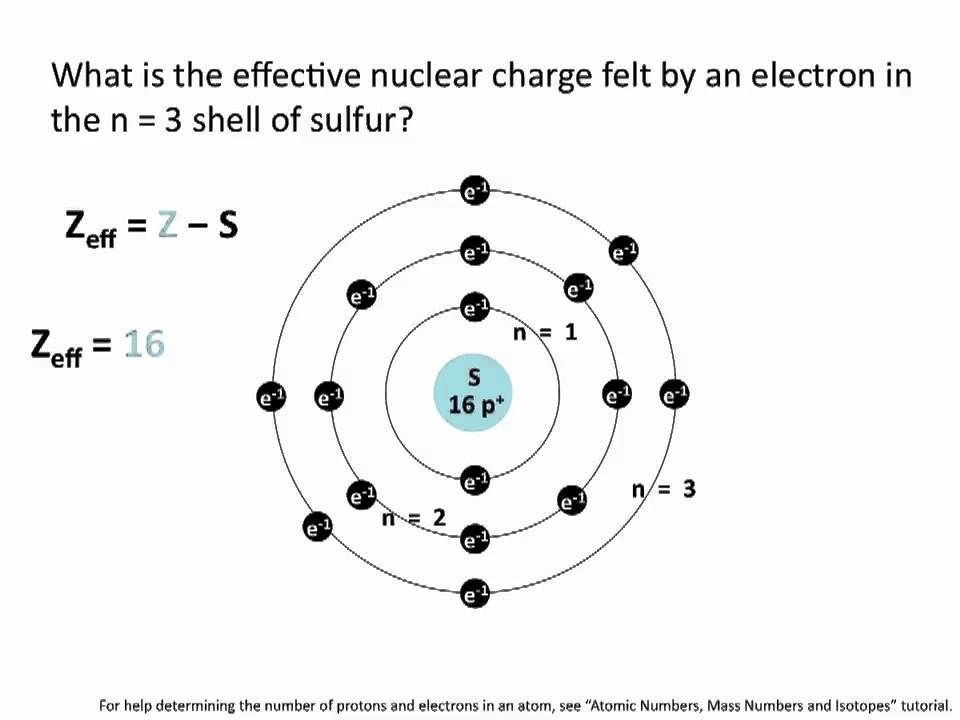
This chemistry video tutorial explains how to calculate the effective nuclear charge of an electron using the atomic number and the number inner shell electr. Increasing effective nuclear charge experienced by the electrons in the n 3 electron shell K Mg P Rh Ti What information do I need to answer this question. Z eff ζ n.
Higher the Effective Nuclear Charge ZEff greater the attractive force which results in electrons being pulled closer to the nucleus. Thus there is a decrease in the effect of nuclear charge. What is the effective nuclear charge for K.
Pitchdiameter ratio Increased pitch increases water. Effect of Core Lattice Geometry on k Assume 2-3 Uranium Vary fuel pin pitchdiameter ratio Calculate η ε p f kas function of. When k-effective is equal to 1 the assembly is called critical if k-effective is less than 1 the assembly is said to be subcritical and if k-effective is greater than 1 the assembly is called supercritical.
Due to the screening effect there is a decrease in the force of attraction on the electron in the valence shell towards the nucleus. P718 Using only the periodic table arrange the following atoms in increasing radius a Cs K Rb b In Te Sn c P Cl Sr. Effective Nuclear Charge of K 220.
The term effective is used because the shielding effect of negatively charged electrons prevent higher orbitals from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. Keff Ratcliffe a Welsh musician and the former bassist for the American band Pretty Boy Floyd.
Electrons that are closer to the nucleus which are referred to as inner or core electrons effectively cancel some of the attraction of outside or valence electrons to the nucleus. Z eff Z - S. Zeff is better known as the effective nuclear charge.
The term effective is used because the shielding effect of. Effective Nuclear Charge. Effective nuclear charge the charge an electron experiences after accounting for the shielding due to other electrons increases from left to right across a given period thus an electron in a 2p orbital of a nitrogen atom experiences a greater Zeff 383 than an.
Effective nuclear charge is the net charge that an outermost shell electron experiences. See all 7 effective nuclear charges. This reduced nuclear charge is called effective nuclear charge is denoted by Z eff.
The effective nuclear charge is the difference between the actual nuclear charge and the screening. F Increases resonance escape. The effective nuclear charge may be approximated by the equation.
ε Decreases thermal utilization. Higher the Shielding Constant S greater the repulsive force between valence and inner core electrons which results in valence electrons pushed away from the nucleus. The effective multiplication factor k eff may be expressed mathematically in terms of the infinite multiplication factor k and two additional factors which account for neutron leakage during neutron thermalization fast non-leakage probability and neutron leakage during neutron diffusion thermal non-leakage probability by following equation usually known as the six-factor formula.
Also the electron or multi-electron takes into account the number of. The effective nuclear charge is then the net electric field that a particular electron experiences. The average number of neutrons that cause new fission events is called the effective neutron multiplication factor usually denoted by the symbols k-effective k-eff or k.
An abbreviation for the reactivity coefficient k-effective written as k eff the effective neutron multiplication factor within an assembly of fissile material in nuclear reactor theory. Effective nuclear charge refers to the charge that the outermost valance electron have. The effective nuclear charge experienced by an electron is also called.
P kreaches maximum value at. The main difference between nuclear charge and effective nuclear charge is that the value of the effective nuclear charge is always a lower value than that of the nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons.
What is Zeff K.

Pin On Missiles Mortars And Rpgs

Nuclear Magnetic Resonance Volume 40 Nuclear Magnetic Resonance Magnetic Resonance Book Of Shadows

This Infographic Shows How Japanese Nuclear Reactors Nuclear Reactor Nuclear Energy Nuclear History

Cerebral School Notes Bullet Journal Inspiration Studyblr

Chemistry Fsc 2nd Year Solved Mcqs Notes Chemistry Notes Chemistry Solving

The Big Picture This Infographic Was Designed For Marketing But This Is Some Helpful Data About Schools Acro Infographic Marketing Big Picture School Reviews

Here Is Some College Hunks Tips And Tricks Here Are 4 Energy Saving Tips For The Holidays Hunks Ch Energy Saving Tips Save Energy Energy Saving Devices

Importantquestionsforclass12chemistrychapter8 Class12chemistry Chemistry Chemistry Education Chemical Equation

Telemedicine Software Cost Technologies And Development Timeline Existek Blog In 2021 Telemedicine Development Software

The Shielding Effect And Effective Nuclear Charge Introduction To Chemistry Electron Configuration Effective Nuclear Charge Chemistry Textbook
Effective Nuclear Charge Chemistry Tutorial Youtube Effective Nuclear Charge Chemistry Jokes Chemistry

How Does Atomic Size Effective Nuclear Charge And Hybridization Affect Electronegativity Chemistry In 2021 Effective Nuclear Charge Chemistry Atom

Lim S S Vos T Flaxman A D Danaei G Shibuya K Adair Rohani H Et Al 2012 A Comparative Risk Assessment Of Burd Analysis Risk Factors Disease

Sprouht Studies 05 03 17 21 100 Days Of Productivity Sorry For The Super Long Hiatus Ahh I V School Organization Notes Study Motivation School Study Tips
Posting Komentar untuk "K Effective Nuclear"