Effective Nuclear Charge
Effective Nuclear Charge Calculation. Nuclear charge is the total charge of a nucleus.

1 Shielding Effect Effective Nuclear Charge Z Eff Experienced By An Electron Is Less Than T Effective Nuclear Charge Ionization Energy Electron Configuration
At intermediate values of r the effective nuclear charge is somewhere between 1 and Z.

Effective nuclear charge. The effective nuclear charge represented as Z eff and in some cases as Z is the net nuclear charge that an electron experiences when it is in a polyelectronic atom that is it has more than one electron. Effective Nuclear Charge Z eff. The main difference between nuclear charge and effective nuclear charge is that the value of the effective nuclear charge is.
The effective nuclear charge often symbolized as Zeff or Z is the net positive charge experienced by an electron in a multi-electron atom. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. Z eff Z - S.
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. The effective nuclear charge Z eff is the number of protons in a nucleus Z minus the screening constant σ. The effective nuclear charge may be approximated by the equation.
Effective nuclear charge refers to the charge that the outermost valance electron have. At r 0 the positive charge experienced by an electron is approximately the full nuclear charge or Z e f f Z. So understanding how it is generated from the formula above will help to give you an intuitive feel for how it works.
Strength of pull of electrons to nucleus Increases down a group a little due to less shielding effect more diffuse core electrons and increasing protons with no change in valence electrons and across the group due to more. The amount of positive charge experienced by any individual electron is the effective nuclear charge Z_eff. The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom.
Introduction to Effective Nuclear charge. Answer 1 of 11. D is the smallest.
Effective nuclear charge is the net charge that an outermost shell electron experiences. This chemistry video tutorial explains how to calculate the effective nuclear charge of an electron using the atomic number and the number inner shell electr. Electronegativity has a huge effect in organic chemistry as we will see.
The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. Because the electrons feel the highest effective nuclear charge Z7 and they are the closest to the nucleus ie. Nuclear charge is the total charge of a nucleus.
Effective Nuclear Charge Example. Z eff Z - σ. This effect called the shielding effect describes the decrease in the attraction between an electron and the nucleus in any atom with more than one electron shell.
Electrons in an atom can shield each other from the pull of the nucleus. The effective nuclear charge is the attractive force of the protons in the nucleus of an atom on an electron after the repulsive force of the atoms electrons is factored out. ENC effective nuclear charge of protons in nucleus of shielding inner shell electrons ENC Calculations.
Electrons are drawn towards the nucleus of the atom and are found in orbitals that fill up in a predictable fashion. Where Z is the atomic number and S is the number of shielding electrons. In this topic we are going to discuss the effective nuclear charge and how to calculate it.
Z eff Z σ where Z eff is the effective nuclear charge Z is the actual nuclear charge and σ is the shielding constant where the shielding constant is greater than zero but smaller than Z. More precisely it is the electric charge that the nucleus of a hypothetical atom would have capable of attracting its only electron with. This video is a crash course on what shielding is what effective nuclear charge is how they are related how they produce specific atomic properties includ.
The main difference between nuclear charge and effective nuclear charge is that the value of the effective nuclear charge is. This will also be equal to the atomic number of the atom. Effective nuclear charge is the net charge that an outermost shell electron experiences.
How to calculate effective nuclear charge. The effective nuclear charge calculated for such an electron is given by the following equation. For example in lithium Li none of the three electrons feel the full 3 charge from the nucleus see Cartoon.
An effective nuclear charge is defined as the net positive charge of the valence electrons of an atom. You will also learn the periodic trend for effective nuclear charge. In a poly electronic atom the valence electrons are simultaneously attracted to the positively charged nucleus and repelled by the negatively charged electrons.
Total charge of an atom is zeroNucleus being positively charged attract the electrons in the various shell of the atomBut the charge experienced by all the election is not sameBecause of shielding of electronsInner shell electron shield the outer electron from the nucleus he. Creating an analogy to attending a concert. An analogy to getting good seats at a concert A better understanding of ENC might come from.
Also the electron or multi-electron takes into account the number of shielding electrons that surrounds the nucleus. Thus the effective nuclear charge the charge felt by an electron is lesser than the actual nuclear charge Z and can be estimated by the following. First determine the number of protons.
Notice that Z e f f Z only for hydrogen Figure 72. In this video you will learn how to calculate effective nuclear charge. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge.
The screening constant is the portion of the nuclear charge that is screened from the valence electrons by the core electrons. 721 1 Z e f f Z.
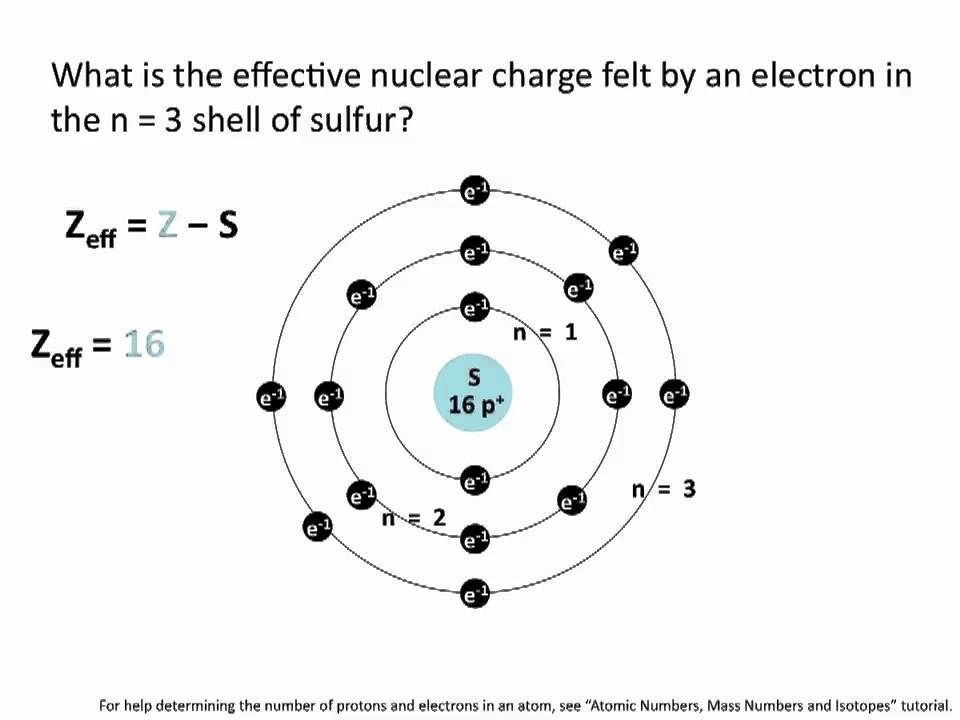
Effective Nuclear Charge Chemistry Tutorial Youtube Effective Nuclear Charge Chemistry Jokes Chemistry

Effective Nuclear Charge Wikipedia Effective Nuclear Charge Nuclear Chemistry

Effective Nuclear Charge Wikipedia Effective Nuclear Charge Nuclear Chemistry

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Electron Configuration Neon Atom

Pin By Faris Barbarossa On الكيمياء Effective Nuclear Charge Chemistry College Chemistry

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration

7 2 Shielding And Effective Nuclear Charge Chemistry Libretexts In 2021 Effective Nuclear Charge Chemistry Neon Atom

Effective Nuclear Charge And The Shielding Effect Effective Nuclear Charge Ionization Energy Electron Affinity

Effective Nuclear Charge Effective Nuclear Charge Happy Students Chemistry

The Shielding Effect And Effective Nuclear Charge Introduction To Chemistry Electron Configuration Effective Nuclear Charge Chemistry Textbook

Shielding Effect And Effective Nuclear Charge Effective Nuclear Charge Chemistry Activities Nuclear

Electronegativity Definition Periodic Trends Examples Importance Electronegativity Difference Digital Kemistry In 2021 Effective Nuclear Charge Chemistry Digital

How Does Atomic Size Effective Nuclear Charge And Hybridization Affect Electronegativity Chemistry In 2021 Effective Nuclear Charge Chemistry Atom

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration
Posting Komentar untuk "Effective Nuclear Charge"