K Effective Nuclear Charge
Nuclear charge is the total positive charge present in the nucleus of an atom. CeO F Ne Na Mg everything changes.

Importantquestionsforclass12chemistrychapter8 Class12chemistry Chemistry Chemistry Education Chemical Equation
Where Z is the atomic number and S is the number of shielding electrons.

K effective nuclear charge. CeLi Na K Rb the effective nuclear charge is similar corresponding to similar chemistry of the valence electrons and you would focus on the valence electrons occupying higher and higher shells. The effective nuclear charge is the net positive charge experienced by valence electrons. The effective nuclear charge may be approximated by the equation.
Effective Nuclear Charge of K 220. According to Slaters Rule effective nuclear charge of Na is x and of K is y respectively. The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom.
What is Zeff K. Higher the Shielding Constant S greater the repulsive force between valence and inner core electrons which results in valence electrons pushed away from the nucleus. Calculate value of x - y.
Comparing neutral atoms in order of atomic number eg. What is the effective nuclear charge for K. Electronic Configuration of K Potassium and its atom has 4 shells in it.
It is the positive charge from a nucleus that an electron feels in an atom with more than one electron present. Comparing atoms in a group eg. The attractive force exerted by a total number of protons on the electrons presents in the outermost orbit is known as an effective nuclear charge.
Increasing effective nuclear charge experienced by the electrons in the n 3 electron shell K Mg P Rh Ti What information do I need to answer this question. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the. The effective nuclear charge often symbolized as Zeff or Z is the net positive charge experienced by an electron in a multi-electron atom.
The main difference between nuclear charge and effective nuclear charge is that the value of the effective nuclear charge is always a lower value than that of the nuclear charge. P718 Using only the periodic table arrange the following atoms in increasing radius a Cs K Rb b In Te Sn c P Cl Sr. The term effective is used because the shielding effect of.
Effective nuclear charge refers to the charge that the outermost valance electron have. Electrons that are closer to the nucleus which are referred to as inner or core electrons effectively cancel some of the attraction of outside or valence electrons to the nucleus. The effective nuclear charge is then the net electric field that a particular electron experiences.
It is simply the product of total number of protons and the charge of one proton atomic number charge of a proton And effective nuclear charge is the nuclear charge experienced by the outershell electron. Z eff Z - S. Zeff Z S where Z is the atomic number and S is the number of shielding electrons.
It can be approximated by the equation. Effective Nuclear Charge of K 220. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons.
Hence 1680 Hence 19 - 1680 220. Higher the Effective Nuclear Charge ZEff greater the attractive force which results in electrons being pulled closer to the nucleus. Z eff ζ n.
Effective nuclear charge is the net charge that an outermost shell electron experiences. Updated February 21 2020. Also the electron or multi-electron takes into account the number of.
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. Zeff is better known as the effective nuclear charge.
By multiplying the Coloumbs law constant k 90 x 109 N m2 C2 by q1 the effective nuclear charge and q2 the charge of the electron and dividing by the radius of the atom squared we can find F which is the force of attraction between the nucleus and the outer electron. See all 7 effective nuclear charges.

Law Of Mass Action 11th Chemistry Chemistry Mass

Non Polar Propter Symmetry Chart Symmetry Polar

Determining Empirical And Molecular Formulas Chemistry Tutorial Youtube Chemistry Education Chemistry Molecular

Chemistry Fsc 2nd Year Solved Mcqs Notes Chemistry Notes Chemistry Solving

Effective Nuclear Charge Chemistry Tutorial Youtube Effective Nuclear Charge Chemistry Jokes Chemistry

Imp Hydroxyquinone Nomenclature Examples Mcat Books Picture

Why Alkanes Are Called Paraffins Paraffin Chemistry Bond

How Does Atomic Size Effective Nuclear Charge And Hybridization Affect Electronegativity Chemistry In 2021 Effective Nuclear Charge Chemistry Atom

The Shielding Effect And Effective Nuclear Charge Introduction To Chemistry Electron Configuration Effective Nuclear Charge Chemistry Textbook

Electronic Configuration For Aluminium Al In 2021 Electron Configuration Chemistry Good Tutorials

Cerebral School Notes Bullet Journal Inspiration Studyblr

Pin By Pieter De Kooker On Chemistry Chemistry Activities Moving
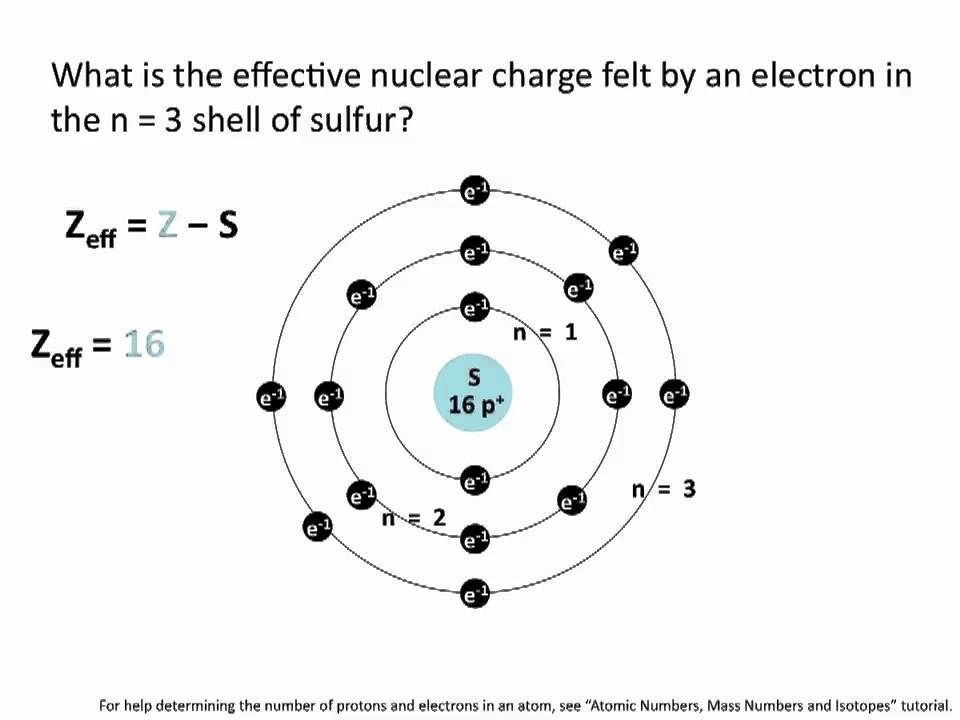
Effective Nuclear Charge Chemistry Tutorial Youtube Effective Nuclear Charge Chemistry Jokes Chemistry

Posting Komentar untuk "K Effective Nuclear Charge"