P Effective Nuclear Charge
The effective nuclear charge often symbolized as Zeff or Z is the net positive charge experienced by an electron in a multi-electron atom. The effective nuclear charge is always less than the total number of protons present in a nucleus due to shielding effect.

Effective Nuclear Charge And The Shielding Effect Effective Nuclear Charge Ionization Energy Electron Affinity
Learn about effective nuclear charge and periodic trends.

P effective nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons. In this topic we are going to discuss the effective nuclear charge and how to calculate it. The effective nuclear charge represented as Z eff and in some cases as Z is the net nuclear charge that an electron experiences when it is in a polyelectronic atom that is it has more than one electron.
The effective nuclear charge is then the net electric field that a particular electron experiences. Answer 1 of 3. Introduction to Effective Nuclear charge.
Hence the effective nuclear charge of the last electron in an atom whose configuration is 1s22s22p63s23p5 is Zefftext text 61 Note. The effective nuclear charge is always less than the total number of protons present in a nucleus due to shielding effect. We view this net electric field as if it results from a single positive charge located at the nucleus called the effective nuclear charge Z eff.
The main difference between nuclear charge and effective nuclear charge is that the value of the effective nuclear charge is. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. It is defined as the effective positive charge felt by an electron in the outer shells of an atom due to the screening effect of electrons in the inner shells.
The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. Write down the electronic configuration. The effective nuclear charge often symbolized as Zeff or Z is the net positive charge experienced by an electron in a multi-electron atom.
The concept of electron shielding in which intervening electrons act to reduce the positive nuclear charge experienced by an electron allows the use of hydrogen-like orbitals and an effective nuclear charge Z_eff to describe electron distributions in more complex atoms or ions. More precisely it is the electric charge that the nucleus of a hypothetical atom would have capable of attracting its only electron with. Effective nuclear charge is behind all other periodic table tendencies.
Effective nuclear charge refers to the charge that the outermost valance electron have. Electrons in an atom can shield each other from the pull of the nucleusThis effect called the shielding effect describes the decrease in the attraction between an electron and the nucleus in any atom with more than one electron shellThe more electron shells there are the greater. This chemistry video tutorial explains how to calculate the effective nuclear charge of an electron using the atomic number and the number inner shell electr.
Effective Nuclear Charge. With atoms the effective nuclear charge refers to the net charge experienced by an atoms outmost electrons. We must remember that the number of electrons in the atom is equal to the atomic number of the element.
The effective nuclear charge acting on an electron in an atom is smaller than the actual nuclear charge Z eff Z because the effective nuclear charge includes the effect of the other electrons in the atom. Effective nuclear charge the attractive positive charge of nuclear protons acting on valence electrons. The effective nuclear charge may be approximated by the equation.
Effective nuclear charge the attractive positive charge of nuclear protons acting on valence electrons. This calculator is based on the Slaters rule of calculating effective nuclear charge. The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom.
We should know about determining the atomic number using the. Effective nuclear charge is behind all other periodic table tendencies. Also the electron or multi-electron takes into account the number of shielding electrons that surrounds the nucleus.
The effective nuclear charge is the net positive charge experienced by an electron in a multi-electron atom. Effective nuclear charge is the net charge that an outermost shell electron experiences. Review atoms the periodic.
Nuclear charge is the total charge of a nucleus. This online chemistry calculator calculates the effective nuclear charge on an electron. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the.
Where Z is the atomic number and S is the number of shielding electrons. Z eff Z - S. Electrons that are closer to the nucleus which are referred to as inner or core electrons effectively cancel some of the attraction of outside or valence electrons to the nucleus.
1s2 2s2 2p6 3s2 3p6 4s1 Total is 19 Effective nuclear charge Zz-s Z19085810010 Z220. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge by the repelling effect of. The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge.
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. Hence the concept of effective nuclear charge is used to get a rough solution.
Follow the steps below to calculate effective nuclear charge by the Slaters rule.
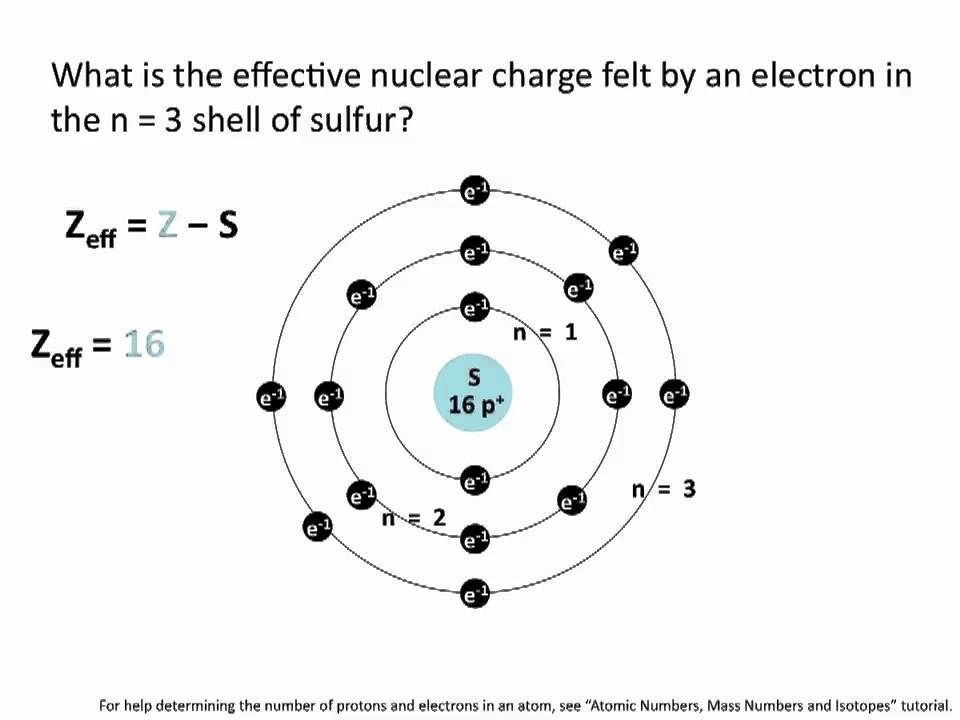
Effective Nuclear Charge Chemistry Tutorial Youtube Effective Nuclear Charge Chemistry Jokes Chemistry

The Shielding Effect And Effective Nuclear Charge Introduction To Chemistry Electron Configuration Effective Nuclear Charge Chemistry Textbook

Pin By Faris Barbarossa On الكيمياء Effective Nuclear Charge Chemistry College Chemistry

Electronegativity Definition Periodic Trends Examples Importance Electronegativity Difference Digital Kemistry In 2021 Effective Nuclear Charge Chemistry Digital

Effective Nuclear Charge Wikipedia Effective Nuclear Charge Nuclear Chemistry

1 Shielding Effect Effective Nuclear Charge Z Eff Experienced By An Electron Is Less Than T Effective Nuclear Charge Ionization Energy Electron Configuration

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration

8 4 Ionization Energy Ionization Energy Chemistry Chemistry Experiments

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration

Effective Nuclear Charge Effective Nuclear Charge Happy Students Chemistry

Shielding Effect And Effective Nuclear Charge Effective Nuclear Charge Chemistry Activities Nuclear

Effective Nuclear Charge Wikipedia Effective Nuclear Charge Nuclear Chemistry

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Electron Configuration Neon Atom

Neon S Atomic Radius Is 38 Pm Effective Nuclear Charge Element Chemistry Electron Affinity
Posting Komentar untuk "P Effective Nuclear Charge"