Effective Nuclear Charge Trend
Is it exact nope but it is in line with the MCATs method of testing these kinds of trends. In the case of the alkali metals Z eff increases from 13 for lithium to 25 for sodium to 35 for potassium.

For All You Chemistry Students Out There If You Re Having Trouble Explaining Periodic Trends It S All Down To The Study Motivation Chemistry Chemistry Jokes
We learned that effective nuclear charge is the positive charge felt by the outermost electrons in an atom.

Effective nuclear charge trend. Z eff Z S. Higher the Effective Nuclear Charge ZEff greater the attractive force which results in electrons being pulled closer to the nucleus. The term effective is used because the shielding effect of negatively charged electrons prevent higher orbitals from experiencing the full nuclear charge of the nucleus due to the repelling effect of inner layer.
The term effective is used because the shielding effect of negatively charged electrons prevents higher orbital electrons from experiencing the full nuclear charge. Besides the formula for calculating the effective nuclear charge of a single electron is as follows. Isotopes that are to the right of the stability line on the chart of the nuclides tend to decay by beta emission - the.
Showing 1 to 0 of 0 rows. Zeff the effective nuclear charge. This is equal to the atomic number Z minus the amount σ that other electrons in the atom shield the given atom from the nucleus.
This results in a trend that in general the effective nuclear charge increases from left to right across any period of the periodic table. Increase across a period due to increasing nuclear charge with no accompanying increase in shielding effect. As you proceed from.
Moving from top to bottom down a column of the periodic table we might expect the elements to have a similar effective nuclear charge as they all have the same number of valence electrons. No matching records found. Effective nuclear charge Effective nuclear charge magnesium 282 net 2 10 inner electron shield 12 protons Valence electron feel a net 12-10 2 Calculate Zeff and atomic radius for Li Effective nuclear charge Zeff 2 1 Calculate Zeff for Li Formula ionization energy 2nd energy level n2 æ Z2 ö IE 1312 ç 2 èn ø æ Z2.
The same treatment by the AAMC goes for electronegativity electron affinity atomic radius and ionization energy. The effective nuclear charge experienced by an electron is also called. Where Z is the atomic number and S is the number of shielding electrons.
The net positive charge from the nucleus that an electron can feel attractions from. Starting from neon 1s 2 2s 2 2p 6 the effective nuclear charge is 8 since 10 protons subtracted by 2 core electrons result in 8. Trends down a group follow from the increasing number of electron shells and the increased distance of the outer electrons from the nucleus.
Effective Nuclear Charge Trend. Z eff Z - S. The major factor is the increasing size.
The screening constant is the portion of the nuclear charge that is. This helps us predict periodic trends. Thus the general trend that would explain this is that Eff Nuc Charge decreases as one goes down a family.
The effective nuclear charge is that portion of the total nuclear charge that a given electron in an atom experiences. The periodic table tendency for effective nuclear charge. The effective nuclear charge is the net charge an electron experiences in an atom with multiple electrons.
The math equation below determines the effective or actual nuclear charge and as mentioned before ENC explains all of the trends in the periodic table. Now lets observe when we go from neon to sodium what occurs to the effective nuclear charge. Effective Nuclear Charge Formula.
Zeff Z S. LEFT RIGHT then Z eff BOTTOM TOP then Z eff Why. Perfect your performance with periodicity.
Z denotes the number of protons existing in the nucleus. Proceeding the group then the n or of shells increases. The core electrons are said to shield the valence electrons from the full attractive forces of the protons in the nucleus.
Trends across a period follow from the increasing number of protons in the nucleus and the decrease in radius. The effective nuclear charge on an electron is given by the following equation. We will discuss effective nuclear char.
The effective nuclear charge often symbolized as Z eff or Z is the net positive charge experienced by an electron in a multi-electron atom. S average amount of. Here we will briefly introduce how the periodic table was developed in order to discuss why there are periodic trends.
The effective nuclear charge may be approximated by the equation. ENC effective nuclear charge of protons in nucleus of shielding inner shell electrons. Decrease down a group although nuclear charge increases down a group shielding effect more than counters its effect.
This helps us predict periodic trends. What are the effective nuclear charge trends. In fact however effective nuclear charge increases slightly as we go down a column because the more diffuse core electron cloud is less able to screen the valence electrons from the nuclear charge.
The effective nuclear charge Z eff is the number of protons in a nucleus Z minus the screening constant σ. We can easily recognize the periodic trends related with this particular property by exploring other periodic properties like atomic radii. Important Trend Terms.
The effective nuclear charge is the actual amount of positive charge experienced by an electron in a polyelectronic atom. Both contributions can be explained by the change in effective nuclear charge. Z eff Z - σ.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. The more shells the greater the distance between the nucleus and electrons and the less of the magnetic force of the positive nucleus. The positive charge of the nucleus that is felt by surrounding electrons of the same atom.
When going down a period in this case period 3 to element 11 sodium posses the quantum numbers of 1s 2 2s 2 2p 6 3s 1. So be able to do this calculation. Higher the Shielding Constant S greater the repulsive force between valence and inner core electrons which results in valence electrons pushed away from the nucleus.

Electronegativity Definition Periodic Trends Examples Importance Electronegativity Difference Digital Kemistry In 2021 Effective Nuclear Charge Chemistry Digital

8 4 Ionization Energy Ionization Energy Chemistry Chemistry Experiments

Periodic Trends Vocabulary Cards Vocabulary Cards Vocabulary Cards

General Chemistry Infographic Atomic Radius Trends In The Periodic Table Chemistry Infographic Education

Periodic Table Trends Ionization Energy Ionic Radius Effective Nuclear Charge

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration
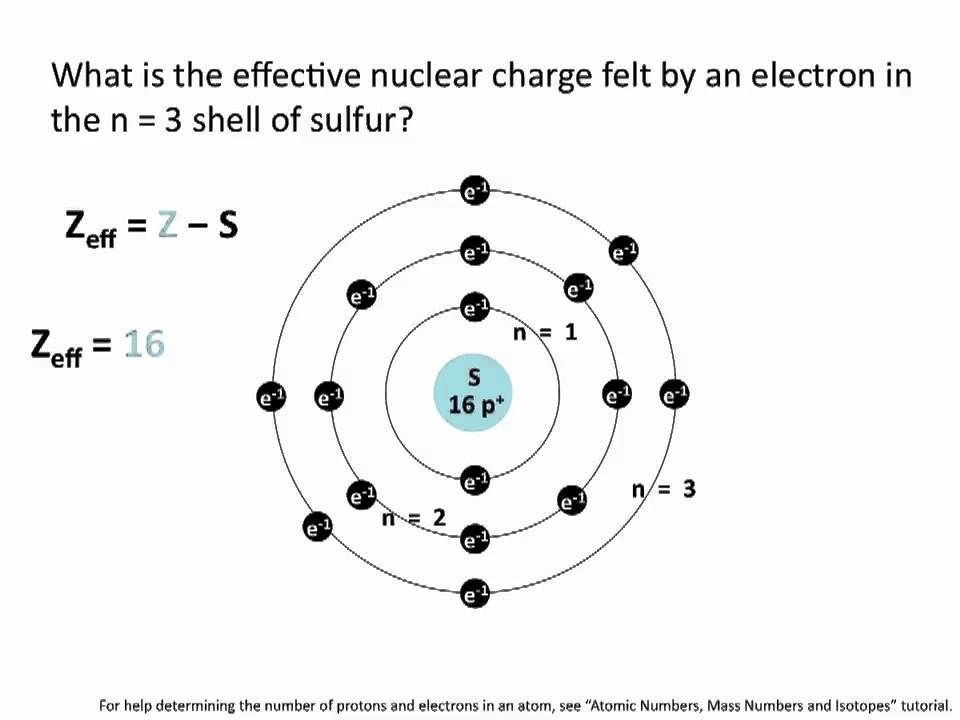
Effective Nuclear Charge Chemistry Tutorial Youtube Effective Nuclear Charge Chemistry Jokes Chemistry

Periodic Table Alkali Metals Group 1a Alkaline Metals Group 2a Transition Metals Group B Metalloids 7 Purp Transition Metal Periodic Table Physical Chemistry

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Electron Configuration Neon Atom

Electronegativity Definition Periodic Trends And Factors Chemistry In 2021 Chemistry Effective Nuclear Charge Periodic Table

Effective Nuclear Charge And The Shielding Effect Effective Nuclear Charge Ionization Energy Electron Affinity

8 6 Periodic Trends In The Size Of Atoms And Effective Nuclear Charge Chemistry Libretexts Effective Nuclear Charge Ionic Radius Electron Configuration

Why That Trend Ionization Energy Mind Mapping Ionization Energy Mind Map Mindfulness

Ketan Periodic Trends Explained Again But Examples Are Given Chemistry Physical Science Ionization Energy
Posting Komentar untuk "Effective Nuclear Charge Trend"